The i-Bio program supports the installation of
Mathieu Hautefeuille
Mathieu Hautefeuille was recruited on September 1, 2021 in a position of Professor of Developmental Biology at Sorbonne University. To support his installation at the IBPS in the Laboratory of Developmental Biology (UMR 7622), he will benefit from an i-Bio financial package. Mathieu has a true interdisciplinary profile: being an Engineer of the microscale by training, he employed microfabrication skills in biomedical applications for the development of microbiosensors and cellular culture devices that can mimic the physicochemical properties of a tissue to help preserve a more relevant cellular identity inside in vitro study devices. He has co-founded and directed the LaNSBioDyT National Laboratory to apply such biomimetis for biomedical solutions for the research laboratory and the clinics at UNAM, Mexico from 2012 to 2020. In parallel to his research activities, his team worked on transferring the know-how to a service platform aimed at all types of users who need to develop organ-on-chip devices and he has trained hundreds of students in mechanobiology, microtechnology and biomechanics. Thanks to his natural position at the interface between Physics, Biology and Medicine, he reconciles the different topics in his lectures, at all levels from Bachelor to Master.
His team focuses on the development of biomaterial and microfluidics-based technology towards microphysiological systems that aim at recapitulating all the natural properties of native tissues in high-fidelity in vitro models, for fundamental research and to develop diagnosis and therapeutic solutions in medicine. The main current project focuses on the study of the role of the multiple stimuli that are responsible for the identity of liver cells in a healthy or pathological tissue. Technological solutions help restore the natural microenvironment of the liver functional units inside a liver-sinusoid-on-a-chip device enabling relevant studies of liver regeneration and pathologies. In particular, the impact of mechanical signals inside the liver lobules is of interest, focusing on the complex multicellular crosstalk occuring at the sinusoid level.
In this endeavor, the team develops multi-layered microfluidic platforms integrating mechanical control of substrate stiffness, luminal pressure and shear stress for multicellular culture. Thanks to collaborations with clinicians and biologists, his work lies at the interface between several disciplines to improve the current models used in research. The team is interested in mechanisms responsible for morphogenesis and maintenance of functional phenotypes, as a response of cells to their direct microenvironment. For this, the aim is to understand the interplay between the physico-chemical cues present inside the tissue that are at play in morphogenesis, homeostasis and diseases to let cultured cells freely and naturally shape morphofunctional tissues in vitro.
Nicolas Biais

Nicolas Biais was recruited on January 1, 2021 on a position of Professor of Cellular Biomechanics at Sorbonne University. To support his installation at the IBPS in the Jean Perrin Laboratory, he will benefit from an i-Bio financial package. Nicolas has a resolutely interdisciplinary profile, having completed a dual training in Physics and Biology throughout his academic education, and holding a PhD at the interface between these two disciplines. He moved to New-York in 2004 to complete his post-doctoral training at Columbia University, then held a position as an Associate Research Scientist. In 2013 he set up his team at Brooklyn College of CUNY. In addition to his research activities, Nicolas has a solid teaching experience at the interface between Physics and Biology where he has successfully put into practice most teaching formats at all levels from Bachelor to Master.
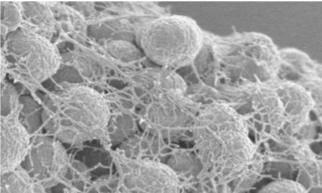
Nicolas Biais is interested in the roles played by the generation of forces by bacteria, in particular by Neisseria gonorrhoeae the infectious agent of gonorrhea, one of the most common venereal diseases. This bacterium has the ability of assembling on its surface long filaments, the Type IV pili, which undergo cycles of elongation and retraction and can be found in many other bacteria. Thanks to these properties of those ubiquitous bacterial appendages, bacteria can generate mechanical forces on their surroundings and use them to move, exchange DNA, colonize surfaces and form biofilms in interaction with other bacteria of the same species, to compete with other bacterial species, and to interact with the human host cells that they infect. In depth studies of the forces generated by Neisseria gonorrhoeae bacteria carry the promise of both fundamental understanding of the role of physical interactions among bacteria and development of innovative therapeutic strategies.