3 Interdisciplinary collaborative projects have received an i-Bio grant in 2023 :
- Onnik Agbulut (UMR8256-IBPS) & Thierry Dufour (Laboratoire de Physique des Plasmas, École Polytechnique)
“Deciphering the Effects of COld atmospheric Plasma on survival and functionality of CARdiomyocytes from patients suffering dilated cardiomyopathy (DECOPCAR)” - Antoine Danon (UMR7238-IBPS) & Raphaël Jeanneret (Laboratoire de Physique de l’ENS)
“Understanding and adapting collective behaviors for optimal use of the model alga Chlamydomonas” - Stéphane Nédelec (UMR-S1270-IFM) & Benoit Sorre (Physico Chime Curie)
“Engineering complex in vitro models of human embryogenesis”
Deciphering the Effects of COld atmospheric Plasma on survival and functionality of CARdiomyocytes from patients suffering dilated cardiomyopathy (DECOPCAR)
Dilated cardiomyopathy (DCM) is a progressive and debilitating disease associated with mutations in several genes. Today, no efficient treatment, pharmacological approach or surgery can revert DCM. Novel research leading to the development of treatments are therefore strongly needed. Cold atmospheric plasmas (CAP) appear as a relevant approach to treat abnormal cardiomyocytes [1]. Indeed, CAP polarized by high voltage pulses and operated in ambient air can generate pulsed electric fields as well as a large variety of active species, especially radicals and reactive oxygen species [2]. Combining these electro-chemical properties are expected to induce adaptive cellular remodeling as well as endogenous repair mechanisms leading to better survival and functionality of cardiomyocytes from the DCM patient. The objective of the DECOPCAR is to study the effects of CAP on cardiomyocytes derived from the patients, characterized by morphological, molecular, functional and metabolic dysfunctions.
[1] Maria Kitsara, Gaëlle Revet, Jean-Sébastien Vartanian-Grimaldi, Alexandre Simon, Mathilde Minguy, Antoine Miche, Vincent Humblot, Thierry Dufour, Onnik Agbulut. Cyto- and bio-compatibility assessment of plasma-treated polyvinylidene fluoride scaffolds for cardiac tissue engineering. Front Bioeng Biotechnol, 2022, 10:1008436.doi: 10.3389/fbioe.2022.1008436
[2] Thomas VON Woedtke, Anke Schmidt, Sander Bekeschus, Kristian Wende, Klaus-Dieter Weltmann. Plasma Medicine: A Field of Applied Redox Biology. In Vivo, 2019, 33(4):1011-1026. doi: 10.21873/invivo.11570.
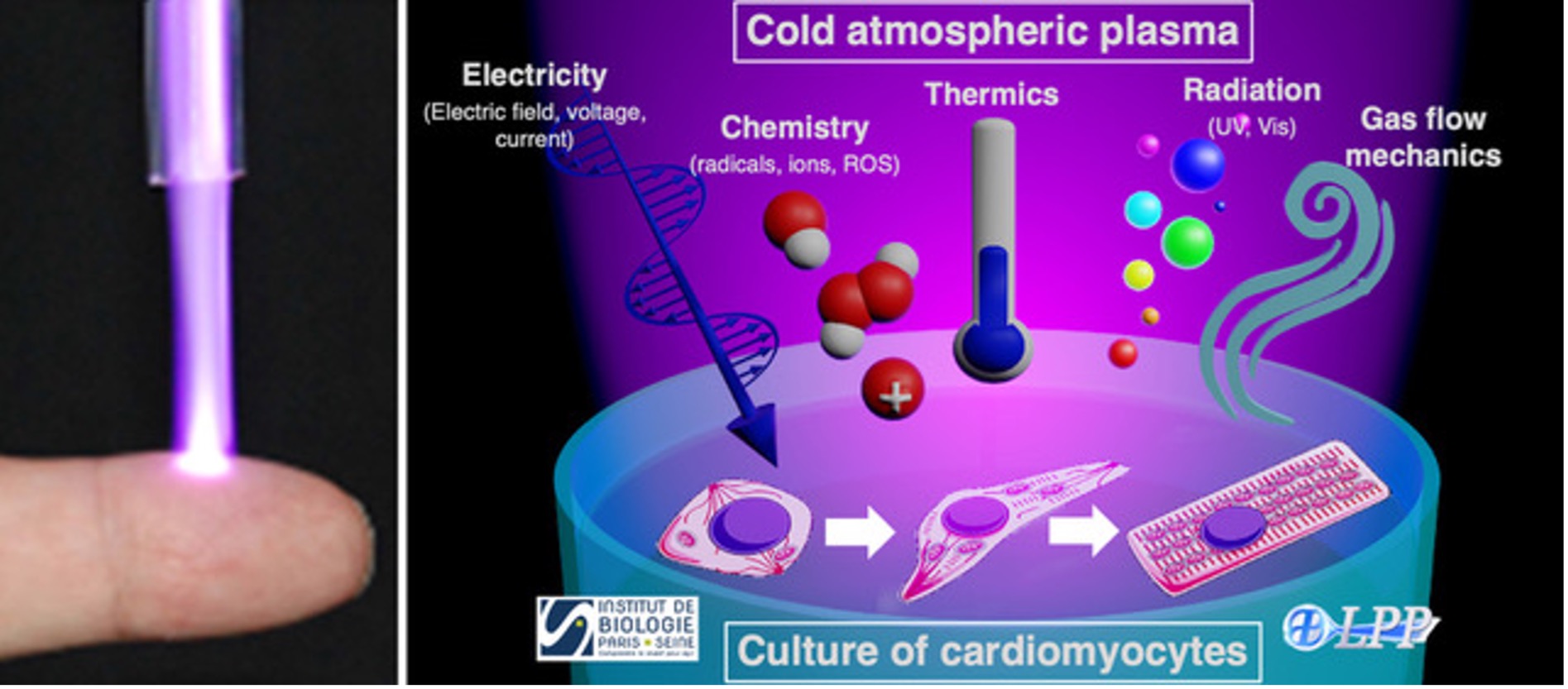
Right: Main properties of Cold atmospheric plasma (CAP) interacting with cardiomyocytes in culture.
Onnik AGBULUT
Biological Adaptation and Ageing (UMR 8256, Inserm ERL U1164), Institut de Biologie Paris-Seine (IBPS)
Team : Stem Cells, Cardiovascular Pathophysiology and Biotherapies
Email: onnik.agbulut@sorbonne-universite.fr
Thierry DUFOUR
Laboratoire de Physique des Plasmas (UMR 7648), Sorbonne Université
Team : Low temperature plasmas
Email: thierry.dufour@sorbonne-universite.fr
Understanding and adapting collective behaviors for optimal use of the model alga Chlamydomonas
We have discovered a new collective behavior of the photosynthetic green alga Chlamydomonas reinhardtii, which upon exposure to various stress conditions form very large aggregates where cells are protected. We have generated the socializer mutant family that aggregate spontaneously and identified the first regulators of aggregation [1]. Our interdisciplinary project aims at coupling biology and physical models to analyze in detail how the cells coordinate to form multicellular structures. From a fundamental point of view our research could lead to a better understanding of the transition to multicellularity, since aggregation is described as an intermediate step in this process. From a more applied point of view, we will use the aggregation regulators that we have identified to allow the harvesting of the cells by a simple filtration system, since this step is for the moment limiting in terms of money and energy to envisage the industrialization of biofuel production in Chlamydomonas.
[1] de Carpentier, F. et al. (2022) How abiotic stress-induced socialization leads to the formation of massive aggregates in Chlamydomonas. Plant Physiol DOI: 10.1093/plphys/kiac321
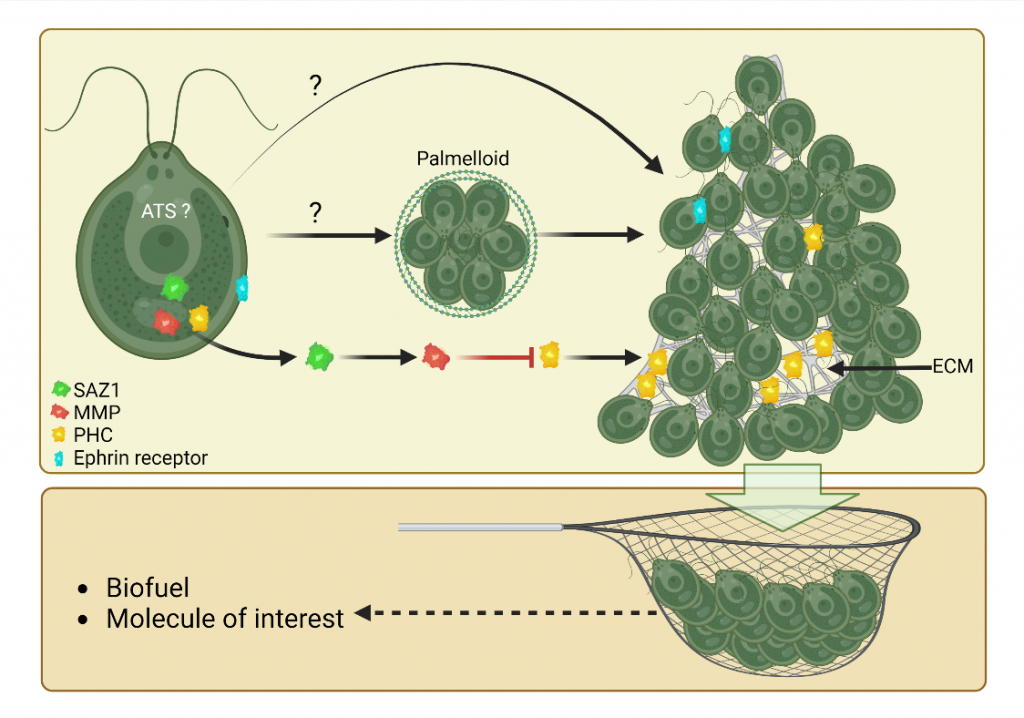
Antoine DANON
Laboratory of Computational and Quantitative Biology (UMR7238), Institut de Biologie Paris-Seine (IBPS).
Team : Synthetic and Systems Biology of Microalgae
Email: antoine.danon@upmc.fr
Raphael JEANNERET
Laboratoire de physique de l’Ecole Normale Supérieure (UMR8023).
Team : Multiscale Physics of Living Systems
Email: raphael.jeanneret@phys.ens.fr
Engineering complex in vitro models of human embryogenesis
The emergence of sensory-motor circuits depends on the generation of distinct spatial organized progenitor domains along the dorso-ventral axis of the caudal neural tube. During embryogenesis, patterns of cell types are largely determined by opposite gradients of signaling molecules. We will thus engineer new microfluidic devices compatible with organoid development in which stable anti-parallel gradient of signaling molecules can be generated and manipulated. We will apply this approach to in vitro generated human neural tube and somites to favor the generation of in-vivo like dorso-ventral pattern of cell types, a patterning process necessary to form functional sensory-motor circuits. Beside the engineering of human sensory-motor circuits, we will develop a general strategy to rationalize the development of human organoid and embryoid models.
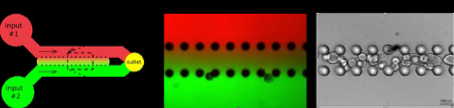
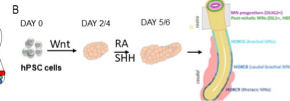
Stéphane NEDELEC
Institut du Fer à Moulin (UMR-S 1270)
Team : Stem cells and Neurodevelopment
Email : stephane.nedelec@inserm.fr
Benoit SORRE
Physico Chimie Curie (UMR168), Institut Curie
Team: Dynamic Control of signaling and gene expression
Email : benoit.sorre@curie.fr
