3 Interdisciplinary collaborative projects have received an i-Bio grant in 2021
- Duprez Dephine UMR7622-IBPS
Legallais Cécile UMR7338-UTC
Click here to discover the project -> In vitro engineering of myotendinous junction
- Mangin Jean-Marie UMR8246-IBPS
Debregeas Georges UMR8237-IBPS
Click here to discover the project -> Dissecting how excitable ependymal cells participate to locomotor rhythms using an all-optical approach
- Weil Dominique UMR7622-IBPS
Gueroui Zoher UMR8640-ENS
Click here to discover the project -> Engineering artificial liquid-liquid phase transition in cells to understand the specificity and function of membrane-less organelles
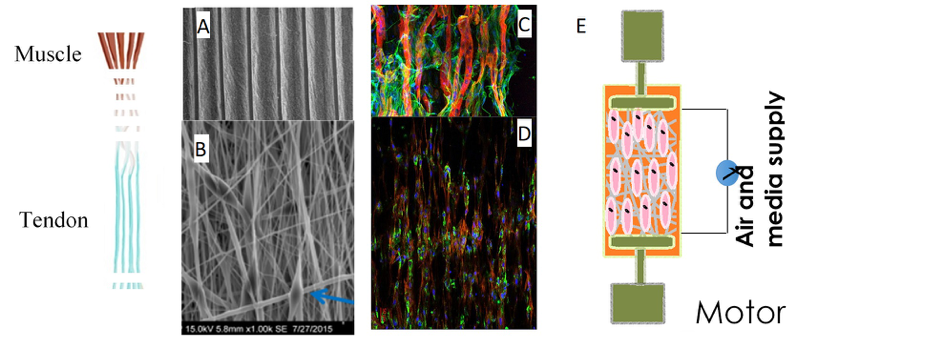
In vitro engineering of myotendinous junction
The myotendinous junction (MTJ) is a key component of the locomotion system as it is at the interface of two major components of the musculoskeletal system, muscle and tendon. MTJ is a key component of force transmission. Despite MTJ key function, the mechanisms underlying MTJ formation are poorly understood. We have evidenced a novel mechanism for the establishment of the MTJ in vivo, with an unexpected recruitment of tendon fibroblasts to myotubes at the MTJ [1]. This novel mechanism leads us to reconsider the cellular basis of MTJ formation. The objective of the project is to take benefit of the knowledge on MTJ formation in vivo to engineer an in vitro MTJ [2]. We will develop a co-culture system of myoblasts and tendon fibroblasts on electrospun biomaterials submitted to cyclic stretching. MTJ is a target tissue affected in tendon injuries and muscle dystrophies, highlighting the interest to understand MTJ formation to develop strategies for MTJ reconstruction.
[1] Esteves de Lima J, Blavet C, Bonnin MA, Hirsinger E, Comai G, Yvernogeau L, Delfini MC, Bellenger L, Mella S, Nassari S, Robin C, Schweitzer R, Fournier-Thibault C, Tajbakhsh S, Relaix F, Duprez D (2020). BMP signalling directs a fibroblast-to-myoblast conversion at the connective tissue/muscle interface to pattern limb muscles
bioRxiv 2020.07.20.211342; doi: https://doi.org/10.1101/2020.07.20.211342
[2] Beldjilali-Labro M, Garcia Garcia A, Farhat F, Bedoui F, Grosset JF, Dufresne M, Legallais C. (2018) Biomaterials in Tendon and Skeletal Muscle Tissue Engineering: Current Trends and Challenges
Materials (Basel). 2018 Jun 29;11(7):1116. doi: 10.3390/ma11071116.
Delphine DUPREZ
Developmental Biology Laboratory (UMR7622), Institut Biologie Paris-Seine (IBPS)
Team : Muscle and tendon formation and repair
Email: delphine.duprez@upmc.fr
Cécile LEGALLAIS
Université de Technologie de Compiègne (UTC)
UMR CNRS 7338 BioMécanique et BioIngénierie (BMBI)
Equipe : Cellules, Biomatériaux, Bioréacteurs
Email: cecile.legallais@utc.fr
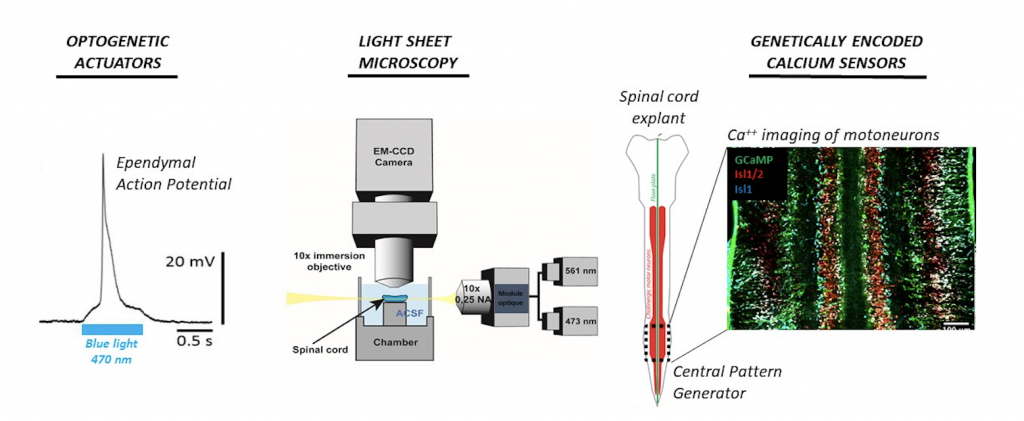
Dissecting how excitable ependymal cells participate to locomotor rhythms using an all-optical approach.
From peristaltic crawling in earthworms to bipedal walking in humans, animal locomotion relies on rhythmic sequences of muscle contractions triggered by a central pattern generator (CPG) in the spinal cord. Despite decades of research, the identity of this CPG remains unclear. We will investigate the original hypothesis that the spinal CPG rely on a group of ependymal cells recently found to generate non-neuronal action potentials [1]. We will use an all-optical approach by expressing genetically-encoded optogenetic actuators and calcium sensors in ependymal cells and spinal neurons. This state-of-the-art neurophysiological approach will be combined to a next-generation functional imaging and optogenetic system allowing us to image large field of tissue in 3D at a high spatiotemporal resolution while performing localized optogenetic stimulation [2]. If successful, the project will be a major milestone in understanding the spinal CPG and how it could be manipulated to restore locomotor function.
[1] Hervé Arulkandarajah, K., Osterstock, G., Lafont, A., Le Corronc, H., Escalas, N., Corsini, S., Le Bras, B., Boeri, J., Czarnecki, A., Mouffle, C., et al. (2020). The floor-plate of His is a non-neuronal electrical conduction pathway. BioRxiv 2020.05.23.111955.
[2] Panier, T., Romano, S.A., Olive, R., Pietri, T., Sumbre, G., Candelier, R., and Debrégeas, G. (2013). Fast functional imaging of multiple brain regions in intact zebrafish larvae using Selective Plane Illumination Microscopy. Front. Neural Circuits 7, 65.
Jean-Marie MANGIN
Neuroscience Laboratory (UMR 8246), Institut Biologie Paris-Seine (IBPS)
Team: Development of Spinal Cord Organization
Email: jean-marie.mangin@inserm.fr
Georges DEBREGEAS
Jean Perrin Laboratory (UMR 8237), Institut Biologie Paris-Seine (IBPS)
Email: georges.debregeas@upmc.fr
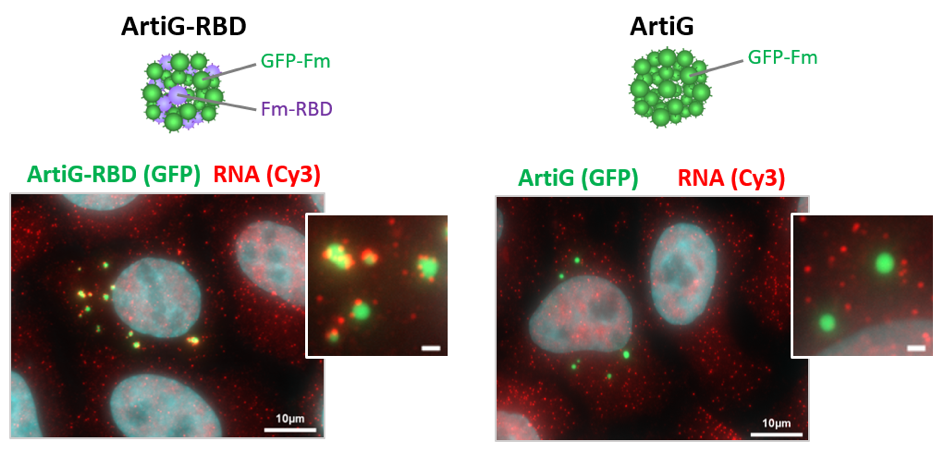
Engineering artificial liquid-liquid phase transition in cells to understand the specificity and function of membrane-less organelles
Membrane-less organelles, which are ubiquitous in intracellular organization, include a variety of ribonucleoprotein (RNP) granules. While the fast-emerging field of phase transitions explores how biomolecules condense into such liquid-like micrometric states, the mechanisms that govern the specificity of RNP condensates and how RNP condensation impacts RNA fate is still not understood.
These questions will be addressed through an interdisciplinary approach combining synthetic biology and cellular biology. To bypass the complexity of RNP granules, we will use synthetic biomolecular assemblies called ArtiGranules, equipped with tunable RNA binding domains to mimic RNP condensates in cells [1]. We will analyze their protein and RNA composition following purification by FAPS, a method previously set up for P-bodies [2], and investigate their consequence on the translation and decay of recruited mRNAs.
[1] Garcia-Jove Navarro M, Kashida K, Chouaib R, Souquere S, Pierron G, Weil D, and Gueroui Z. RNA is a critical element for the sizing and the composition of phase-separated RNA-protein condensates. Nature Com. 2019. https://doi.org/10.1038/s41467-019-11241-6
[2] A Hubstenberger, M Courel, M Bénard, S Souquere, M Ernoult-Lange, R Chouaib, Z Yi, JB Morlot, A Munier, M Fradet, M Daunesse, E Bertrand, G Pierron, J Mozziconacci, M Kress, and D Weil. P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol Cell 2017. https://doi.org/10.1016/j.molcel.2017.09.003
Dominique WEIL
Developmental Biology Laboratory (UMR7622), Institut Biologie Paris-Seine (IBPS)
Email : dominique.weil@upmc.fr
Zoher GUEROUI
Department of Chemistry, Ecole Normale Supérieure
Email : zoher.gueroui@ens.fr
